Abstract
BACKGROUND: Pregnant women with SCD have an increased risk of both maternal and fetal complications, including preeclampsia, venous thromboembolism, and cardiomyopathy, as well as an increased risk of intrauterine growth restriction, preterm birth, low birth weight, and fetal demise. While in general children with a history of intrauterine growth restriction are at risk for abnormal neurodevelopment as well as later obesity and metabolic syndrome, the long-term developmental outcomes of children without SCD but exposed to maternal SCD are unknown. Better data about the long-term developmental outcome for children of mothers with SCD will provide guidance about the utility of disease modifying therapy during pregnancy. The objective of this study is to explore outcomes for children born to mothers with SCD using data from the Boston Birth Cohort (BBC).
METHODS: The BBC is a prospective birth cohort established in 1998 at the Boston Medical Center (BMC) that recruits preterm cases (born at less than 37 weeks gestation) and full term controls. The BBC enrolls predominantly urban, low-income minority mothers and their children 24-72 hours after delivery at the BMC and follows the children from birth up to age 21 years. Data is collected on maternal preconception medical conditions, pregnancy complications, and pregnancy exposures through structured interviews at the time of enrollment and medical record extraction. Data on child development is collected from medical records and structured interviews performed by trained study staff at their scheduled pediatric appointments. Approximately 38% of study participants self-identify as black/African American, 22% as Hispanic, 19% as Haitian, and 8.5% as white. From maternal electronic medical record data capturing 147,827 emergency room and outpatient visits contributed by 5,972 subjects, we identified all women with ICD-9-CM codes for sickle cell disease (282.60-64, 282.68, 282.69, 282.41, 282.42) and probable (two or more 282.62 codes) sickle cell anemia. We then linked maternal and child data on the basis of a unique family-level study ID. Information on child development was obtained from electronic medical record data, based on the presence of relevant ICD-9-CM hospital diagnosis codes from pediatric outpatient, inpatient, and emergency room visits.
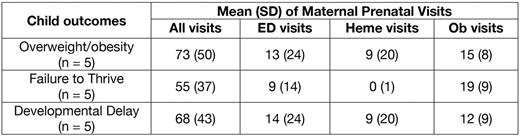
RESULTS: We identified 93 women who had at least one of the ICD-9 codes for SCD. For this report we have included only those forty-one women who were coded at least twice with a 282.62 diagnosis (Hemoglobin SS disease with crisis). Six (15%) had poor fetal growth during their pregnancy, while eight (20%) were diagnosed with preeclampsia or antepartum hypertension. Among these mothers with available questionnaire and medical record extraction data (n = 38), 7 (18%) delivered their children before 37 weeks gestation, and 6 (16%) had children weighing less than 2500 grams at birth. Twenty mothers had children who received pediatric care at the Boston Medical Center, with ages ranging from 2 to 9 years of age; one was diagnosed with SCA, while 19 are unaffected carriers. Among the 19 children without SCD with available follow-up data, 5 (26%) carried a diagnosis code of overweight/obesity; their mothers were seen for an average of 73 medical visits (SD 50) during their pregnancy. Five children, without any overlap with the children diagnosed with obesity, were diagnosed with failure to thrive; while not statistically significantly different, their mothers had fewer medical encounters (mean 55, SD 37). Five children were diagnosed with delayed milestones, two of whom were late preterm (35 and 33 weeks gestation); two of these children were diagnosed with obesity and two with failure to thrive.
DISCUSSION: This exploratory, descriptive study represents the first data on long-term outcomes of children without SCD who were exposed to maternal SCD in utero . While we are limited by small sample size, finding that 26% of children born to mothers with SCD have developmental delay is a significant concern. This compares to the overall cohort where 12% of control children and 39% of preterm cases have been found to have developmental delay. Future studies will include medical record review of mothers to identify additional mothers with confirmed SCD in the cohort and exploration of additional predictors of these concerning outcomes.
Lanzkron: Prolong: Research Funding; HRSA: Research Funding; Bayer: Research Funding; Global Blood Therapeutics: Research Funding; Pfizer: Research Funding; PCORI: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal